Optimize Your Biotherapeutic Protein & Peptide Chromatography
The world of biotherapeutic characterization is undergoing a revolution. Development of evermore complex protein-based therapeutics places rigorous demands on analytical technologies.
Scientists require powerful and flexible solutions to fully characterize biotherapeutic proteins.
Because of their innovative architecture, the Thermo Scientific™ Vanquish™ Flex UHPLC systems were engineered to build your drug pipeline, catering specifically for the demanding analytical requirements that these molecules impose.
Vanquish Flex UHPLC systems have been designed and manufactured to exacting engineering standards to be truly
"Built for Biopharma."
Download BrochureVanquish Flex UHPLC delivers CONFIDENCE, with a fully biocompatible flow path and proven compliance. Achieve unprecedented PERFORMANCE in retention time stability, sensitivity and separation efficiency with the widest range of column chemistries for biotherapeutic proteins. Demand VERSATILITY through seamless integration with market-leading mass spectrometry, fluorescence and charged aerosol detection. Gain operational SIMPLICITY with easy, freely available, one-click workflows via AppsLab and tool-free Thermo Scientific™ Viper™ fittings.

Vanquish UHPLC System: Best in UHPLC Column Thermostatting to Fit All Needs
Download
Vanquish UHPLC System: Best in UHPLC Column Thermostatting to Fit All Needs
The required technique to control the actual separation temperature is often underestimated and, in many cases, not well understood in UHPLC. Thermostatting has to be effective, but must not compromise the achievable column efficiency. On the other hand, it is very important to account for successful method transfer between different instruments. The Vanquish UHPLC system provides an entirely new and unique combination of active eluent pre-heating and advanced column thermostatting modes.
Download
Vanquish UHPLC System: How Solvent Delivery Technology Can Improve Confidence in Peak Identification and Quantification
Download
Vanquish UHPLC System: How Solvent Delivery Technology Can Improve Confidence in Peak Identification and Quantification
With the higher pressure capabilities of the Vanquish UHPLC system, the use of very long columns and column chains with sub-2 micron particles are no longer a challenge. Thus peak capacities beyond the common UHPLC range are now achievable, and even more complex matrices can be resolved. Additionally, this enhances requirements for reliable peak identification, with regards to higher retention time reproducibility, to maintain the level of data quality.
Download
What Efficient Temperature Control Can Teach Us in Liquid Chromatography
Download
What Efficient Temperature Control Can Teach Us in Liquid Chromatography
Each good chromatographer wants to implement proper temperature control. For this purpose, column thermostats have evolved from simple units just providing a fixed temperature to high-tech devices. Elevated temperatures, well above the boiling point of the mobile phase, become more and more applied. Consequently, mobile phase pre-heating prior to entering the column as well as post-column mobile phase cooling prior to entering the detector becomes mandatory. In addition, viscous heating is a much discussed topic in Ultra High Performance Liquid Chromatography (UHPLC). When pressures increase to values far above 400 bar, friction between column and mobile phase generates high amounts of viscous heat and, thereby, an additional temperature increase inside the column.
Download
Why Have High Performance When You Can Have Ultra-High Performance?
Read Online
Why Have High Performance When You Can Have Ultra-High Performance?
Liquid chromatography plays an extremely important part in the development and production of biotherapeutic drugs. From separation of large quantities of antibodies in cell culture media to minute quantities of product from biological fluids during clinical studies, there is a growing need to be able to separate and measure these substances. The Russian botanist Mikhail Tsvet is considered to have invented the chromatographic technique (link to Lab Manager article) when he reported separations of different plant pigments into a series of colored bands on a packed column (see image below). He called this technique chromatography. HPLC became popular in the 1960s and 1970s, with UHPLC becoming popular in the 1990s. A clue as to the longevity of the technique is that the HPLC meeting (link to 2015 conference) is now in its 42nd year!
Read Online
Antibodies Attack! What Happens When Biotherapeutics Go Bad?
Read Online
Antibodies Attack! What Happens When Biotherapeutics Go Bad?
It’s well known that biotherapeutic drugs (also known as biologics) are more difficult to produce consistently than our old friends, small molecule drugs. And, manufacturers spend untold amounts of money trying to make sure that each batch of biologics not only performs exactly the same way but also meets the strict regulatory requirements for consistency. By the way, here is a very informative 9-min video by Professor DeMasi at MCPHS University, Boston (USA), titled, Therapeutic Applications of Monoclonal Antibodies, that is worth a watch.
Read OnlineBuilt for Biopharma: State-of-the-Art UHPLC for Characterizing Biotherapeutics
WatchBuilt for Biopharma: State-of-the-Art UHPLC for Characterizing Biotherapeutics
In this webinar, we will discuss the development of LC methods that are optimized to meet the requirements of modern biotherapeutics characterization labs.

Antibodies as Therapeutics
Watch
Antibodies as Therapeutics
Antibodies are an attractive choice as therapeutics for a number of reasons such as their antigen specificity, modes of action and their difficulty to be easily copied when coming off patent. Our own immune system has yielded us a powerful weapon which we can utilise to fight disease. However, as we produce these antibody therapeutics in vitro we bypass the immune systems careful in vivo control mechanisms to ensure antibody efficacy and safety. Therefore there are strict requirements to fully and comprehensively characterise antibody therapeutics to certify no changes to the antibody have occurred during its production which could affect its function. In this webinar, we shall look deeper in to antibodies themselves, how they develop, their structure, functions and modes of action. Why antibodies are such powerful biopharmaceuticals and how they are currently being utilised will also be discussed with relevant examples. This first section of the webinar will end with an overview of potential modifications that can occur to an antibody and the effects they have.
Watch
'Ultra' Biotherapeutic Characterisation
Download
'Ultra' Biotherapeutic Characterisation
With the current market value for biopharmaceuticals standing at more than $150 billion (1), the need for higher throughput, more in-depth characterisation techniques for biologics is accentuated. Can recent technological advances in UHPLC meet the practical and regulatory requirements of the biotherapeutic development chain?
Download
AppsLab Library - Biopharma Methods
Learn More
AppsLab Library - Biopharma Methods
The AppsLab Library of Analytical Applications is a fully searchable online, analytical method repository where you can find applications with detailed method information, chromatograms and related compound information. All the information needed to run, process and report the analysis is available in ready-to-use eWorkflows.
Learn More
BioLC Column Chemistries FlipBook
View Online
BioLC Column Chemistries FlipBook
View Online
Pittcon 2016 – Day 3 – Adventures in Biopharma
Read Blog Online
Pittcon 2016 – Day 3 – Adventures in Biopharma
Read Blog Online
Liquid Chromatography as the Future Replacement for Gel Electrophoresis
Read Blog Online
Liquid Chromatography as the Future Replacement for Gel Electrophoresis
Read Blog Online
Five Instrument Requirements to Separate Your HPLC from the Status Quo
Read Blog Online
Five Instrument Requirements to Separate Your HPLC from the Status Quo
Read Blog Online
Challenging the Status Quo at HPLC 2016
Read Blog Online
Challenging the Status Quo at HPLC 2016
Read Blog Online
Behavioural Economics, Black Swans and HPLC
Read Blog Online
Behavioural Economics, Black Swans and HPLC
Read Blog Online
Can LC/LC-MS Ever Replace Immunoassays?
Read Blog Online
Can LC/LC-MS Ever Replace Immunoassays?
Read Blog Online
LPG or HPG Pump for LC-MS – We Need Your Vote!
Read Blog Online
LPG or HPG Pump for LC-MS – We Need Your Vote!
Read Blog Online
Biopharmaceutical Production in Your Garden Shed
Read Blog Online
Biopharmaceutical Production in Your Garden Shed
Read Blog OnlineProtein Aggregate Analysis
Regulatory bodies typically impose a limit on the acceptable degree of protein aggregation that a biotherapeutic may exhibit, and therefore the confirmation of protein aggregate levels is vital throughout the drug development and production process. Our solutions for protein aggregation detection are rapid and reliable, giving you confidence in your ability to identify and separate higher order structures.
Contact Us
Dimers, trimers and further higher order structures affect clinical efficacy and must be characterized, typically by size exclusion chromatography (SEC).
Our recommended SEC column, MAbPac SEC-1, uses spherical, fully porous ultrapure silica, typically with a 5 µm particle size and multiple column lengths.
Vanquish Flex UHPLC provides high sensitivity, high resolution SEC under non-denaturing conditions and in high-salt mobile phases.
Compliant, simple, data management and reporting is performed using Thermo Scientific™ Chromeleon™ CDS.
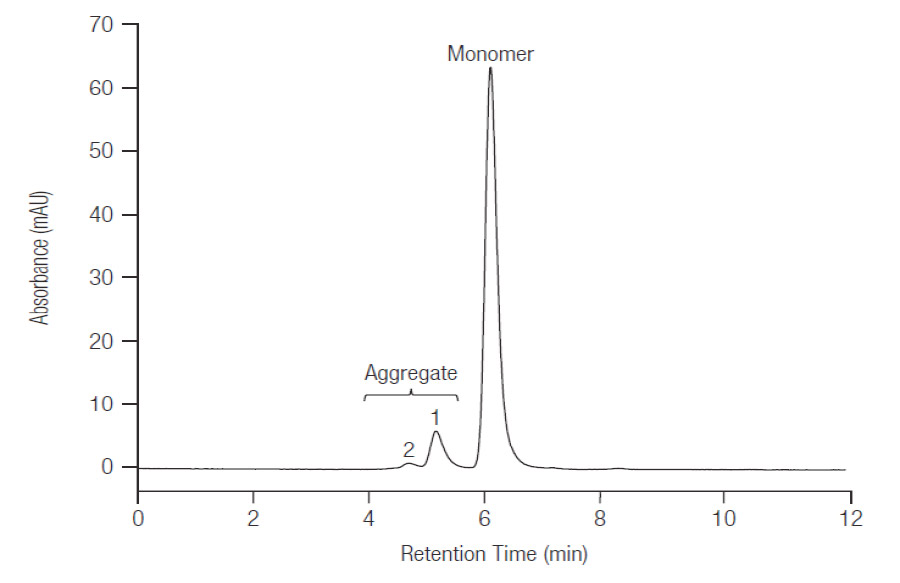
Baseline separation of trastuzumab monomer and oligomer with simultaneous detection of main compound and aggregates using MAbPac SEC.
Aggregation screening requires a powerful and flexible UHPLC. But column selection also plays a critical part in this workflow. MAbPac SEC has a proprietary hydrophilic bonded layer that results in minimal non-desired interactions between the stationary phase and the biomolecules. Its nonmetallic and biocompatible PEEK housing eliminates metal contamination from the column.
Resources
SEC-MS with Volatile Buffers for Characterization of Biopharmaceuticals HIC as a Complementary, Confirmatory Tool to SEC for the Analysis of mAb Aggregates 'Ultra' Biotherapeutic Characterisation AppsLab Library - Aggregate Methods Avoiding Deadly Aggregates in Bio-therapies: Part 1 of a Monoclonal Antibody Characterization SeriesRelated Products
Thermo Scientific™ MAbPac™ SEC-1 Size Exclusion LC Column Thermo Scientific™ MAbPac™ HIC-10 LC ColumnGlycan Analysis
Even small changes in the type, composition or linkage of attached glycans can alter biotherapeutic efficacy, meaning that correct description is vital. We have a range of powerful and innovative chromatography solutions for released, labeled and non-labeled glycan analysis by HPLC and UHPLC.
Contact Us
Multiple characterization techniques are possible; as free gycans, as glycopeptides, or as intact proteins.
Glycans can be labelled for easier detection, a typical example being fluorescence labelling for detection using a fluorescence detector (FLD).
GlycanPac columns have innovative mixed-mode surface chemistry, combining WAX and reversed phase or HILIC functionality for separation according to charge, size, and in the case of GlycanPac AXR-1, isomerism.
Vanquish Flex UHPLC system is fitted with a fluorescence detector, which is perfect for labelled glycans at the lowest concentrations. It also has a CAD detector, ideal for unlabelled glycans.
MS techniques for glycan proteins should ideally be high resolution accurate mass (HRAM) for maximum quantification and structural elucidation.
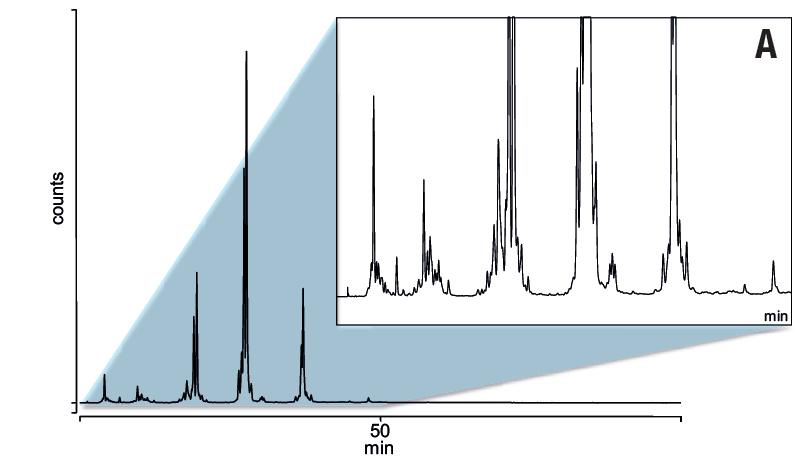
Six replicates of separation of N-glycans from fetuin using GlycanPac AXR-1 column.
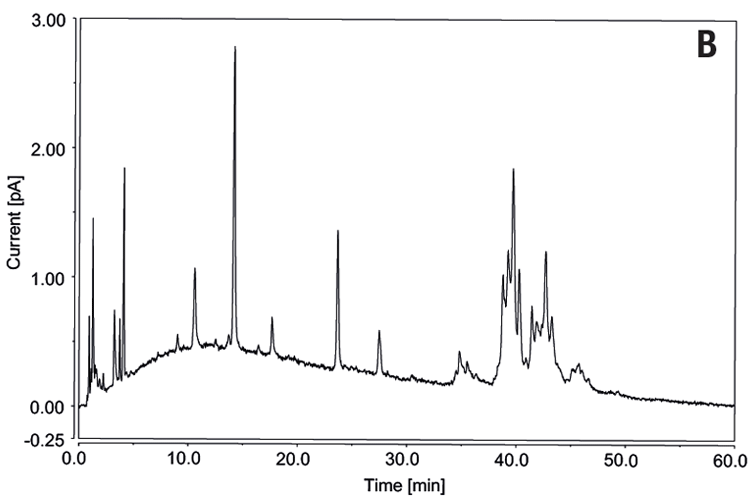
Fetuin O-linked native glycans analyzed by UHPLC-CAD using GlycanPac AXH-1.
Detection of labelled glycans using FLD is well established, an example of this is shown for N-linked glycans in the upper of the two chromatograms (A). It is also desirable to separate and detect glycans without prior derivatization. The Vanquish Flex UHPLC Charged Aerosol Detector (CAD) has consistent analytical has consistent analytical response independent of chemical structure and a dynamic range of four orders of magnitude. CAD data of unlabelled O-linked glycans is shown in the lower of the two chromatograms (B).
Resources
Label-Free Analysis by UHPLC with Charged Aerosol Detection of Glycans Separated by Charge, Size, and Isomeric Structure UHPLC Analysis of 2-Aminobenzamide-Labeled Glycans with the Vanquish Flex UHPLC System Label-free N-Glycan Profiling (Poster) Label-free O-Glycan Profiling (Poster) Label-free N-Glycan Profiling (Poster Brief)Label-free N-Glycan Profiling
In this poster brief, David Thomas describes label-free analysis and separation of N-glycans using UHPLC and charged aerosol detection (CAD). Separation of N-glycans by charge, size and isomeric structure can be achieved without convoluted labelling methodologies.
Label-free O-Glycan Profiling
In this poster brief, David Thomas describes label-free profiling of O-glycans using UHPLC and charged aerosol detection (CAD). Separation of O-glycans by charge, size and isomeric structure can be achieved without convoluted labelling methodologies.
Webinar: The Sweet Revolution in Glycan and Antibody Separations
This webinar discusses the difficulties and also the current technologies used to maximize glycan separation capabilities and structural elucidation.
Related Products
Thermo Scientific™ GlycanPac™ AXR-1 LC Column Thermo Scientific™ Accucore™ Vanquish™ C18+ UHPLC ColumnCharge Variant Analysis
Protein charge homogeneity can have a significant effect on the structure, stability, binding affinity, and efficacy of a biotherapeutic drug. Ion exchange chromatography (IEX) is often used for protein charge variant profiling, either by salt gradient or more recently by pH gradient.
Contact Us
Antibody variants may have different charge states, meaning separation according to charge is required, usually by IEX.
Our dedicated buffer kits allow set up of pH gradients with ease.
MAbPac SCX-10 is a strong cation exchange column designed specifically for high-resolution separations of antibodies and associated variants by IEX.
Vanquish Flex UHPLC is ideal for routine, high throughput ion exchange chromatography with generic and steep gradients, being fast, robust and reliable and having twice the sample capacity of many UHPLC systems.
Compliant, simple, data management and reporting is performed using Thermo Scientific™ Chromeleon™ CDS.
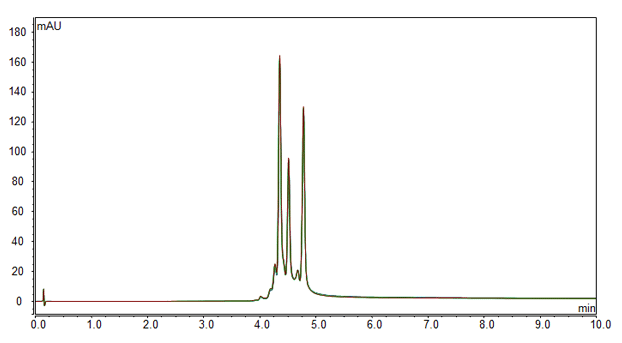
pH gradient ion exchange of monoclonal antibody (overlay of six runs).
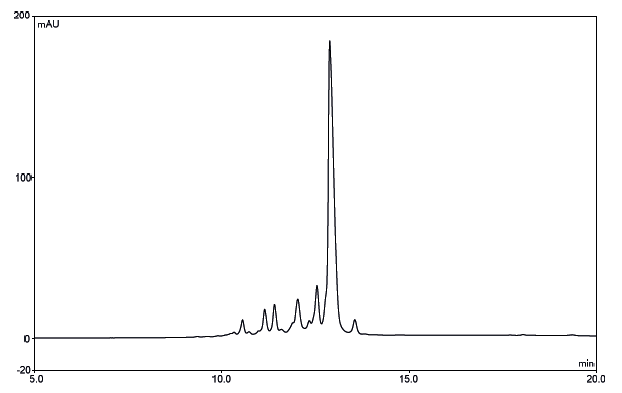
Optimized salt gradient for monitoring deamidation of ribonuclease with ProPac WCX.
Identification and separation of charge variants is performed rapidly and reliably. The use of a pH gradient-based system can be more robust and less challenging than a salt-based system. The unique nonporous pellicular resin used in MAbPac SCX-10 provides high resolving power, permitting the separation of monoclonal antibody variants that differ by as little as one charge.
Resources
Reversed-Phase Separation of Intact Therapeutic Antibodies Using the Vanquish Flex UHPLC System High Salt Gradient Analysis of Post-Translational Modifications - Deamidation Monitoring 'Ultra' Biotherapeutic Characterisation Utilizing the Native Fluorescence of Monoclonal Antibodies for the Sensitive Detection of Charge Variants Transferring a Method for the Characterization of PTMs from the UltiMate 3000 BioRS System to the Vanquish Flex UHPLC System AppsLab Library - Charge Variant MethodsRelated Products
Thermo Scientific™ pH Buffer Thermo Scientific™ MAbPac™ SCX-10 LC ColumnPeptide Mapping
Peptide mapping protocols that detail the entire protein (100% sequence coverage) are required to prove molecular structure as well as determine post-translational modifications (PTMs). Complex protein digests require high peak capacity and high-resolution separations. Our peptide mapping workflow solution includes a fast, reproducible protein digestion protocol and powerful yet simple peptide mapping software, with Vanquish Flex UHPLC at its heart.
Contact Us
Prior to peptide mapping, proteins must be digested into their constituent peptides, typically using an enzyme.
Thermo Scientific™ SMART Digest™ kits are designed for biopharmaceutical applications that require highly reproducible, sensitive and fast analyses, with digests taking <60 minutes, for high-throughput routine workflows..
UV and MS detection are both widely used in peptide mapping. UV is often used in routine environments and is the workflow described here.
Thermo Scientific™ Acclaim™ VANQUISH™ C18 UHPLC Column is an excellent choice of column, it has an ultrapure silica substrate with extremely low metal content to minimize tailing effects and deliver the sharp, symmetrical peak shapes needed in this workflow.
Vanquish Flex has a very high pressure flow path and sample pressurization prior to injection to ensure high peak capacity, retention time stability and peak area precision, ideal for peptide mapping applications.
MS techniques for intact proteins should ideally be high resolution accurate mass (HRAM) for maximum quantification and structural elucidation.
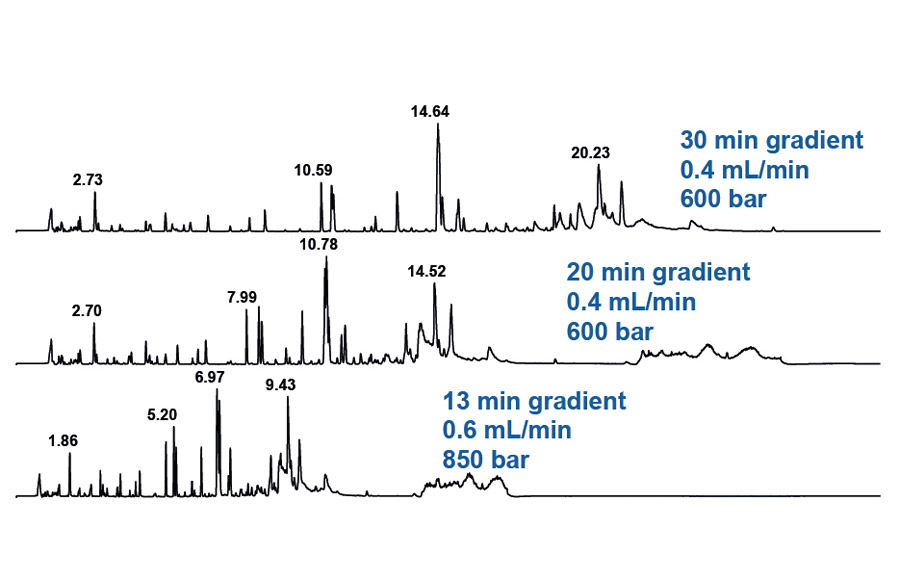
Total ion chromatograms obtained from peptide mapping experiments of rituximab applying gradient lengths of 30, 20 and 13 minutes.
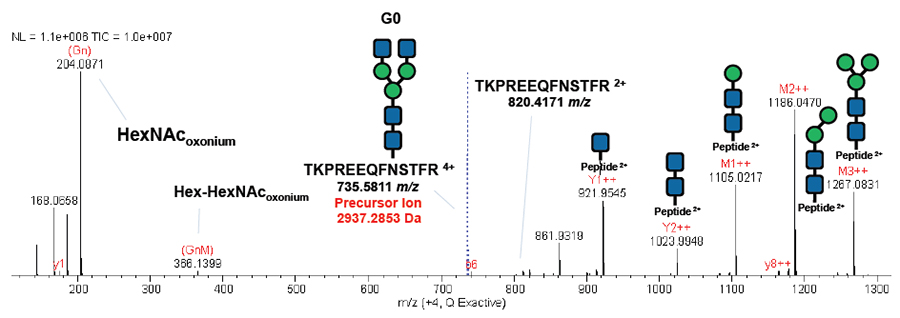
MS/MS spectrum of the rituximab glycopeptide aa 290-302 (TKPREEQFN*STFR, *=G0) with the typical fragmentation pattern: the two oxonium ions 204 (HexNAc), 366 (Hex-Hex-NAc), and the sequence ladder of the fragmented glycan attached to the intact peptide.
Coupling the Vanquish UHPLC with Orbitrap™ mass spectrometers and Thermo Scientific™ BioPharma Finder™ Software provides accurate, in-depth characterization and relative quantification of biotherapeutic peptides for ultimate peptide mapping confidence.
Resources
Reliable Results in Peptide Mapping Using the Vanquish Flex UHPLC System LC-UV-MS Peptide Mapping Development for Easy Transfer to LC-UV QA/QC High throughput Peptide Mapping by UHPLC-MS (Poster) 'Ultra' Biotherapeutic Characterisation New Integrated Informatics Solution for Protein Biotherapeutics Characterization High throughput Peptide Mapping by UHPLC-MS (Poster Brief)High throughput Peptide Mapping by UHPLC-MS
In this poster brief, Stephane Houel, Biopharma Product Marketing Specialist, describes a high throughput peptide mapping workflow using the Thermo Scientific Vanquish UHPLC system and Thermo Scientific Q Exactive HF mass spectrometer.
Get Smart with Protein Digestion
Related Products
Thermo Scientific™ SMART Digest™ Kit Thermo Scientific™ Acclaim™ VANQUISH™ C18 UHPLC Column Peptide Mapping Workflows: Fast, confident and more reliable peptide mapping BioPharma Finder Software: Workflow driven informatics for complete biotherapeutic characterizationIntact Mass Analysis
There remains a need to characterize biotherapeutic proteins in an intact form, particularly where there is likelihood of structural information such as isomerism that may not be observed by other techniques. It is also important in some of the novel biotherapeutic classes such as antibody drug conjugates (ADCs). Our range of Thermo Scientific™ MAbPac™ HIC columns are particularly well suited to the characterization of ADCs, allowing DAR determination in minutes.
Contact Us
mAbs and ADCs require thorough characterization, including glycoforms, higher order structures and PTMs.
MAbPac™ RP is a new reversed phase column that uses a resin based on hydrophobic supermacroporous 4µm polymer particles. It is ideal for the efficient separation of protein molecules with very low carry over.
UHPLC is the most effective technique to ensure separation of higher order structures and unwanted interferents.
MS techniques for intact proteins should ideally be high resolution accurate mass (HRAM) for maximum quantification and structural elucidation.
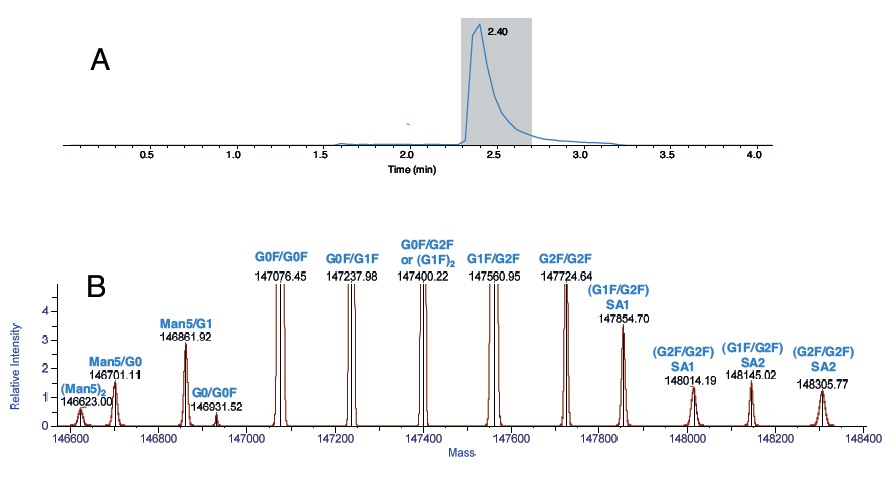
MS analysis of 100 ng of the mAb Rituximab, depicting (A) LC chromatogram using Thermo Scientific MSPac™ DS-10 Desalter Cartridge, and (B) the deconvoluted spectrum and annotated glycoforms.
The Vanquish Flex UHPLC system readily hyphenates to the full range of Thermo Scientific™ mass spectrometers including gold standard Orbitrap™ mass analyzer instruments. The Thermo Scientific™ Q Exactive BioPharma™ Platform offers a High Mass Range (HMR) mode of operation, ideally suited for the analysis of intact protein complexes, such as mAbs, and ADCs in the native or denatured state.
Reproducibility of protein separation is as important as resolution and sensitivity. Vanquish Flex UHPLC produces excellent separation reproducibility giving you the confidence you need.
Resources
High Salt Gradient Analysis of Post-Translational Modifications - Deamidation Monitoring (Application Brief) SEC-MS with Volatile Buffers for Characterization of Biopharmaceuticals (Application Note) Reversed-Phase Separation of Intact Therapeutic Antibodies Using the Vanquish Flex UHPLC System (Application Note) Fast Online Desalting of mAbs Using a Reversed-Phase Desalting Cartridge for LC-MS Analysis (Application Note) Influence of Column Temperature on Reversed-Phase Chromatography of an Intact Antibody (Technical Note) High Temperature, High Throughput Reversed Phase Separation of Intact Monoclonal Antibodies (Poster Note) Enabling Mass Spectrometric Analysis of Intact Proteins in Native Conditions on A Hybrid Quadrupole-Orbitrap Mass Spectrometer (Poster Note) Full Characterization of Heterogeneous Antibody Samples Under Denaturing and Native Conditions on a Hybrid Quadrupole-Orbitrap Mass Spectrometer (Poster Note) Complete Characterization of a Cysteine-linked Antibody-Drug Conjugate Performed on a Hybrid Quadrupole-Orbitrap Mass Spectrometer with High Mass Range (Poster Note) New Integrated Informatics Solution for Protein Biotherapeutics Characterization (Poster Note) AppsLab Library - Intact Mass MethodsRelated Products
Thermo Scientific™ MAbPac™ HIC-20 HPLC Column Thermo Scientific™ MAbPac™ RP Column Thermo Scientific™ MSPac™ DS-10 Desalter Cartridge Q Exactive BioPharma: The complete BioPharma characterization solution BioPharma Finder Software: Workflow driven informatics for complete biotherapeutic characterization
SelectScience: Biopharmaceutical Method Transfer in Regulated Environments
Read Online
AnalyteGuru: How to Stand Out from the Biopharmaceutical CMO Crowd
Read OnlineRequest a Vanquish™ Platform Demo
Want to see the platform in person? Register for a demo to see how the Vanquish UHPLC platform can help solve your toughest chromatographic challenges!